Abstract
Background R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) is the standard of care for newly diagnosed and untreated diffuse large B-cell lymphoma (DLBCL); however, approximately 30-40% of patients relapse or become refractory. Furthermore, the 5-year overall survival (OS) is lower in high-risk DLBCL patients than in low-risk patients. Co-administration of lenalidomide (LEN) with R-CHOP improved both progression-free survival (PFS) and OS in the ECOG-ACRIN E1412 trial but not in the Phase III ROBUST study, in which a lower dose of LEN was used, despite a positive trend in 2-year PFS rate in a subgroup analysis of patients with high-risk disease. POLARIX (NCT03274492) evaluated a modified regimen substituting polatuzumab vedotin for vincristine (pola-R-CHP), showing a modest improvement in PFS without OS benefit between treatment arms, indicating an unmet clinical need remains.
Tafasitamab, an Fc-enhanced, humanized, anti-CD19 monoclonal antibody, in combination with LEN was granted accelerated approval in the US (2020) and conditional/accelerated approval by the EMA (2021) and other regulatory authorities for the treatment of adult patients with relapsed/refractory DLBCL not otherwise specified, including DLBCL arising from low-grade lymphoma, and who are ineligible for ASCT. It is a preferred regimen in the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for this setting.
CD19 and CD20 are broadly expressed on the surface of DLBCL cells, and a treatment strategy targeting both of these B cell surface molecules may limit target evasion in patients exhibiting low CD20 expression and reduce resistance to R-CHOP. Additionally, LEN is an immunomodulatory drug with antineoplastic activity and enhances antibody-dependent cellular cytotoxicity with tafasitamab and rituximab. The addition of tafasitamab + LEN to R-CHOP may provide further therapeutic benefit in patients with newly diagnosed and untreated DLBCL.
The Phase Ib First-MIND (NCT04134936), randomized, safety study of R-CHOP + tafasitamab ± LEN in patients with newly diagnosed and untreated DLBCL showed that tafasitamab + LEN does not affect the dosing and scheduling of R-CHOP, with toxicities similar to those expected with R-CHOP alone (ASH 2021; #3556).
frontMIND (NCT04824092) will investigate the efficacy and safety of R-CHOP + tafasitamab + LEN versus R-CHOP alone in previously untreated patients with high-intermediate and high-risk DLBCL.
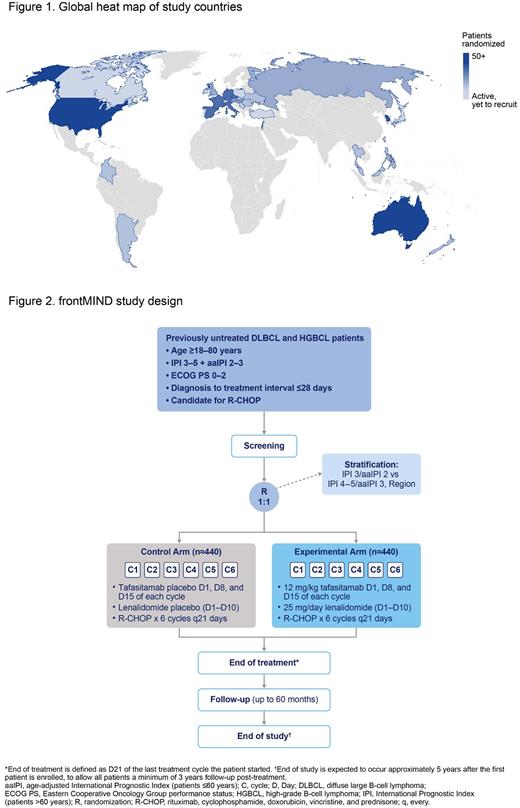
Methods frontMIND is a Phase III, multicenter, randomized, double-blind, placebo-controlled study. As of June 2, 2022, more than 350 study sites have been activated in over 25 countries globally (Figure 1). The study is currently enrolling, with a planned sample size of 880 patients; as of June 6, 2022, more than 50% of the planned sample size have been enrolled in the study. Eligible patients include adults aged 18‒80 years with previously untreated, local biopsy-proven CD20+ DLBCL and high-grade B cell lymphoma (double-hit/triple-hit lymphoma if deemed ineligible for a more aggressive treatment) with IPI score 3-5 (age-adjusted IPI 2-3 if ≤60 years), Eastern Cooperative Oncology Group performance status 0-2, and a time from diagnostic biopsy to start of treatment within 28 days (Figure 2). Patients will be randomized 1:1 to receive six 21-day cycles of either R-CHOP + tafasitamab (12 mg/kg IV, Days 1, 8, and 15) + LEN (25 mg orally, Days 1-10) in the experimental arm or R-CHOP + tafasitamab placebo (0.9% saline solution IV, Days 1, 8, and 15) + LEN placebo (orally, Days 1-10) in the control arm. Patients will be followed for safety and efficacy up to 60 months after the treatment ends. The primary endpoint is investigator-assessed PFS. Secondary endpoints include investigator-assessed event-free survival, OS, safety, and tafasitamab serum concentration (trough and Cmax levels). The sensitivity and specificity of minimal residual disease (MRD) for early detection of disease progression is an exploratory endpoint; further MRD parameters may also be investigated.
The study is funded by MorphoSys AG and conducted with the scientific support of members of the Fondazione Italiana Linfomi and the German Lymphoma Alliance.
Disclosures
Vitolo:Seagen, Genmab, Incyte, Costellation, Bayer, Regeneron: Consultancy; AbbVie, Incyte, Janssen, Gilead Sciences: Speakers Bureau. Nowakowski:Bantam Pharmaceutical: Consultancy; Blueprint Medicines Corporation: Consultancy; Celgene Corporation/Bristol Myers Squibb: Consultancy, Research Funding; Curis, Inc.: Consultancy; Daiichi Sankyo Inc: Consultancy; F. Hoffmann-La Roche Ltd: Consultancy, Research Funding; Genentech, Inc: Consultancy, Research Funding; Incyte: Consultancy; Karyopharm: Consultancy; Kite Pharma Inc.: Consultancy; Kymera Therapeutics: Consultancy; MorphoSys US Inc: Consultancy; NanoString: Research Funding; Ryvu Therapeutics: Consultancy; Selvita: Consultancy; TG Therapeutics: Consultancy; Zai Lab: Consultancy. Burke:Nurix: Consultancy; Roche/Genentech: Consultancy; Morphosys: Consultancy; Kymera: Consultancy; Kura: Consultancy; Epizyme: Consultancy; Bristol Myers Squibbs: Consultancy; BeiGene: Consultancy, Speakers Bureau; AstraZeneca: Consultancy; Adaptive Biotechnologies: Consultancy; Abbvie: Consultancy; SeaGen: Consultancy, Speakers Bureau; TG Therapeutics: Consultancy; Verastem: Consultancy; X4 Pharmaceuticals: Consultancy. Fox:Roche: Other: Travel to scientific congress; Celgene/BMS, Gilead/Kite, Incyte, Janssen, Roche, Takeda: Speakers Bureau; BeiGene: Research Funding; Abbvie, AstraZeneca, Atarabio, Celgene/BMS, GenMab, Gilead/Kite, Incyte, Janssen, Morphosys, Ono, Roche, Takeda: Consultancy. Trneny:Takeda: Consultancy, Honoraria, Research Funding; Zentiva: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Research Funding; MorphoSys: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Gilead Sciences: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Bristol-Myers Squibb: Consultancy, Honoraria. Chiappella:Celgene-Bristol Myers Squibb: Other: lecture fee, advisory board; Clinigen: Other: lecture fee, advisory board; Gilead Sciences: Other: lecture fee, advisory board; Janssen-Cilag: Other: lecture fee, advisory board; Roche: Other: lecture fee, advisory board; Takeda: Other: lecture fee, advisory board; Astrazeneca: Other: lecture fee; Incyte: Other: lecture fee; Novartis: Other: lecture fee; SecuraBIO: Other: advisory board; Ideogen: Other: advisory board. Waldron-Lynch:MorphoSys, US Inc., Novartis AG (immediate family member): Current Employment, Current equity holder in private company. Wagner:MorphoSys: Current Employment, Current holder of stock options in a privately-held company, Other: Travel and accomodation expenses. Pachori:Novartis, MorphoSys AG: Current Employment, Current equity holder in private company. Lenz:Gilead: Consultancy, Honoraria, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; BMS: Consultancy, Honoraria, Speakers Bureau; Bayer: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Research Funding, Speakers Bureau; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Morphosys: Consultancy, Honoraria, Research Funding, Speakers Bureau; Genmab: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria; Constellation: Consultancy, Honoraria; ADC Therapeutics: Consultancy, Honoraria; Miltenyi: Consultancy, Honoraria; Takeda: Honoraria, Speakers Bureau; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; University Hospital Münster: Current Employment.
OffLabel Disclosure:
Tafasitamab is a humanized Fc-modified cytolytic CD19 targeting monoclonal antibody. In combination with lenalidomide (LEN), it received accelerated approval in July 2020 for adult patients with relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL) not otherwise specified (NOS), including arising from lowâ€'grade lymphoma, who are ineligible for autologous stem cell transplant (ASCT). Following FDA approval, we are now evaluating the safety and efficacy of tafasitamab in combination with LEN as an add-on to first-line therapy with R-CHOP versus R-CHOP alone in newly diagnosed patients with high-intermediate and high-risk DLBCL.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal